

To indicate that heat is added, the lattice energy would be expressed as a positive 787 kJ/mol:
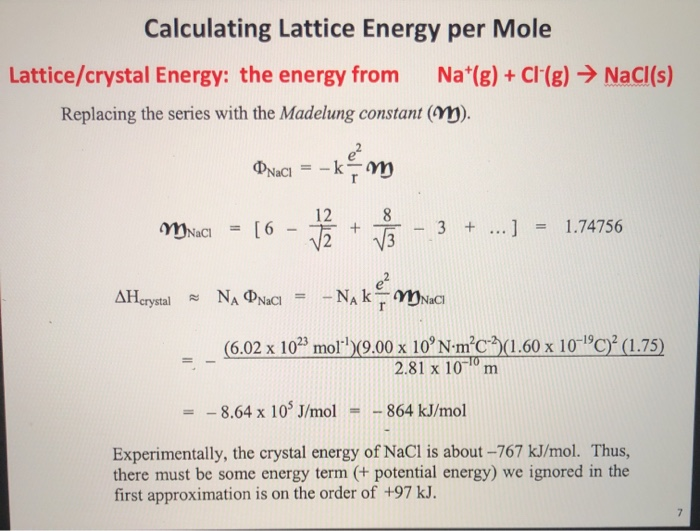
To convert the NaCl solid back to the gaseous ion state, enough heat would have to be added to break the lattice bond. Δ H = – 787 kJ/mol Negative means heat was lost (exothermic) and the reaction resulted in a lower energy state Symbolically the lattice energy is expressed as the change in enthalpy associated with forming the solid lattice: The number is negative and energy is lost as heat. In the case of reacting gaseous sodium cation and gaseous chloride anion to form a lattice of sodium chloride solid, the reaction is expressed symbolically as:īy looking at a table of known values we determine the change in enthalpy or lattice energy to be negative 787 kJ/mol. Lattice Energy is the enthalpy change (Δ H) that accompanies the formation of 1 mole of an ionic solid from its separated gaseous ions. The next term you will need to understand to calculate lattice energy using the Born-Haber Cycle is Lattice Energy itself.ĥ. We discussed the energy required to break the Cl-Cl bond in the discussion of dissociation energy. Remember that Cl normally exists as a diatomic molecule, Cl 2 gas.
#EQUATION OF LATTICE ENERGY OF NACL FREE#
A free radical is an atom with at least one lone electron in its outer shell. That means that the chloride anion, Cl- (g) is more stable than the chlorine free radical, Cl (g). Since heat is released, the reaction is exothermic and the Δ H is a negative number. The electron affinity for chlorine can be found on a table of known electron affinities and is expressed as the delta H:
This increased stability is confirmed by the change in enthalpy. Chlorine needs an electron to complete its octet. Part II begins with a continuation of the discussion on EA: Electron Affinity.Ĭhlorine is happier (more stable) as a charged gaseous ion (g) than a gaseous free radical (g) with an unshared electron. Connect to WiFi to avoid cellular charges for video streaming.īe sure to watch video’s Lattice Energy Part I and Part II before reviewing the transcription.


 0 kommentar(er)
0 kommentar(er)
